Abstract
BACKGROUND: Multiple myeloma (MM) is a hematological malignancy characterized by the uncontrolled growth of clonal plasma cells. High-dose chemotherapy followed by autologous stem cell transplantation (ASCT) is now standard of care for newly diagnosed MM patients with improved outcome. However, high-risk MM patients relapse after 1 to 2 years post-ASCT. Immune checkpoint blockade using nivolumab (Nivo), an anti-PD1 monoclonal antibody or ipilimumab (Ipi), an anti-CTLA4 monoclonal antibody, as single agents have achieved durable responses in patients with advanced solid tumors.
RATIONALE: In a murine melanoma model, Nivo or Ipi monotherapy partially reduced tumor burden, whereas combined Nivo and Ipi therapy eliminated tumor burden. In patients with advanced cancers such as melanoma and non-small lung cancer, combined Nivo+Ipi resulted in superior clinical efficacy compared to patients treated with single agents. Since monotherapy with Nivo did not show an objective response rate in relapsed/refractory MM patients, we hypothesized that high-risk MM patients treated with consolidation therapy consisting of Nivo plus Ipi post-ASCT would achieve a more durable response and improve progression-free survival.
OBJECTIVE: While the objective of the clinical trial focused on safety and efficacy of the combination therapy approach, our study focused on blood immune analysis by flow cytometry, and particularly on the regulatory (Treg) and memory T cell compartments.
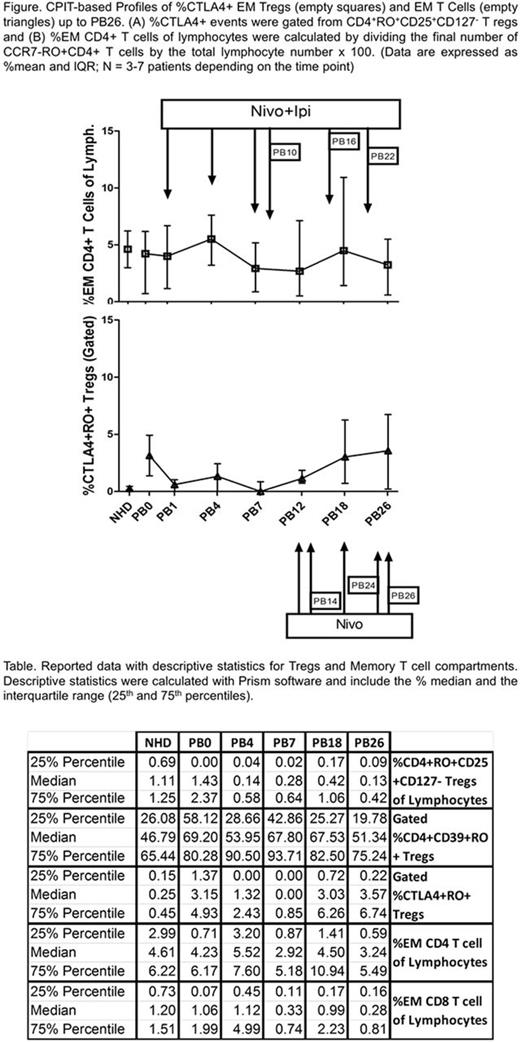
STUDY DESIGN: In a phase Ib-IIA study, patients were grouped into 6 cohorts (7 patients each) with various hematological malignancies. This abstract focuses on results from the transplant-naive high-risk MM group: those carrying 1q amplifications, 1p, 13q, or p53 deletions, high-risk GEP 70 scores, t(4;14), t(14;16) and t(14;20), or hypodiploidy). Peripheral blood (PB) samples obtained from patients at time of initial screening served as a baseline (PB0). The patients underwent hematopoietic progenitor cell (HPC) mobilization (high-dose cyclophosphamide, dexamethasone and etoposide followed by filgrastim), apheresis, conditioning with melphalan 200 mg/m2, and HPC reinfusion. Starting 2-4 wks later, they were administered double therapy (Ipi. 1mg/kg; 6 doses at week 1, 4, 7, 10, 16, 22 and Nivo. 3 mg/kg; 12 doses at week 1, 4, 7, 10, 12, 14, 16, 18, 20, 22, 24, 26). PB was collected just prior to any treatment at various time points (see Figure), and normal healthy donor PB served as population standards for flow cytometry.
PRELIMINARY RESULTS: This information includes the reported % mean + interquartile range data for the high risk MM group up to week 26, given that the study is still on-going. Thus far, the gated % of Tregs CD4+RO+CD25+CD127- decreased throughout the therapy: 1.4%, (PB0), 0.1%, (PB4), 0.3%, (PB7), 0.4%, (PB18), 0.1%, (PB26). Moreover, the CD4+CD39+RO+ (Memory) Tregs initially decreased and then rebounded with double therapy administration: 69%, (PB0), 54% (PB4), 68% (PB7), 68% (PB18), and 51% (PB26). Moreover, the percent of CTLA4+RO+ Tregs was altered after the first combination treatment: 3.2% (PB0), 1.3% (PB4), 0% (PB7), but was restored by PB18 (3.0%) and PB26 (3.6%). Similar results were observed in the absolute numbers of these populations. Furthermore, there was a marked increase in CD4+ effector memory (EM) T cells at PB4 (5.5%) and PB18 (4.5%) compared to PB0 (4.0%). And CD8+ EM T cells were not affected at PB4 (1.1%) and PB18 (1%), compared to PB0 (1.1%). In contrast, both CD8+ and CD4+ central memory (CM) T cell compartments were low at all time points. Interestingly, while CD4+CCR7+RO- terminally differentiated (TEMRO-) T cells did not recover to PB0 levels, the %CD8+CCR7+RO- TEMRO- T cells steadily increased up to PB18, but then decreased by PB26: 1.8% (PB0), 2.4%(PB7), 2.5%(PB18), and 1.2%(PB26). Similar results were observed using the absolute number of cells.
CONCLUSIONS: Combination Nivo+Ipi checkpoint inhibitors can decrease the presence of memory Tregs overall as well as CTLA4+ Tregs. In addition, the approach was able to increase the percentage and absolute numbers of the CD4/CD8 EM T cell compartments, but did not affect the CD4/CD8 CM T cell compartments, thus enabling the immune system to potentially still be able to target cancer cells while in therapy. Finally, the increase in CD8+ TEMRO- T cells were consistent with the exhaustion of longer surviving EM T cells.
Biran: Celgene, Amgen: Consultancy, Speakers Bureau; Takeda: Speakers Bureau. Richter: Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Speakers Bureau. Siegel: Celgene, Takeda, Amgen Inc, Novartis and BMS: Consultancy, Speakers Bureau; Merck: Consultancy. Skarbnik: Gilead: Speakers Bureau; Genentech: Speakers Bureau; Abbvie: Other: Ad board, Speakers Bureau; Novartis: Speakers Bureau; Seattle Genetics: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.